Effective since December 14, 2016
It is now official: after years of consultations and discussions, the new Canadian food labelling regulations, based on the latest scientific evidence, have finally passed legislation. They are in force since the day of their publication in Canada Gazette part II, on December 14, 2016.
Overall, the objective of the government is to make the information on food labels more useful and easier to read. Thus, Canadians would be able to make informed choices about the food they consume, and better tooled to maintain or improve their health.
The regulatory modifications will change the content and aspect of ingredient lists and nutrition labelling. With respect to ingredient lists, the modifications are significant. In terms of content, two major changes are required:
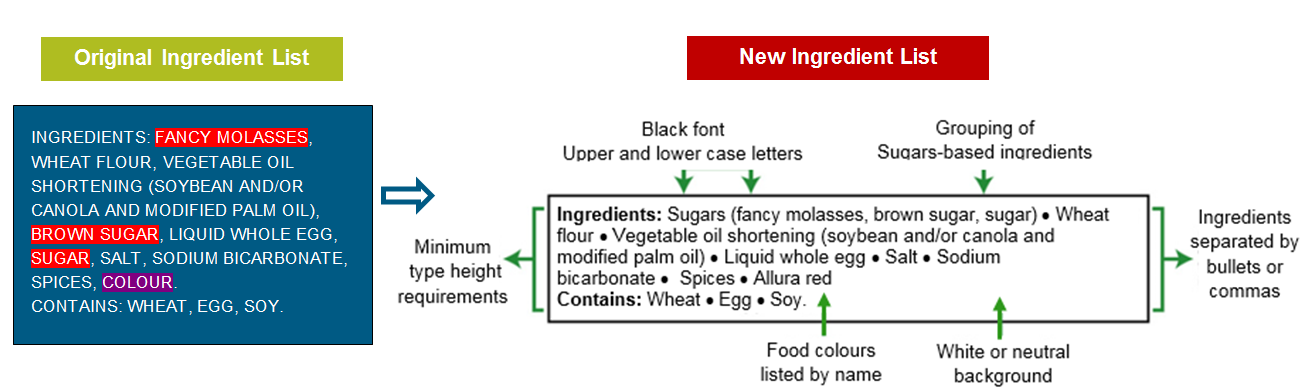
- the grouping of sugars-based ingredients within parentheses after the word “sugars” in the ingredient list; and
- the ungrouping of food colour additives.
Under the new regulations, it will no longer be allowed to group the various food colour additives under the common name “colour”. The idea is to give more information to consumers with allergies or sensitivities to certain food colour additives.
As for the grouping of sugar-based ingredients (e.g. sugar, honey, glucose, fructose, malted barley, fruit juice concentrates, etc.), if the product contains two or more sugar-based ingredients, these will need to be listed individually, but grouped together, within parentheses, after the word “sugars”. The idea is that by grouping all sugar-based ingredients, and listing the combined sugars in the proper descending order of proportion by weight in the finished product, we will have a better sense of the proportion of sugar-based ingredients in the product. Sugar is a nutrient of public health concern, and Health Canada hopes that this new requirement will offer food manufacturers an incentive to reduce the total sugar content of their food products.
Also, several graphic enhancement features are now prescribed in the regulations to improve the legibility of ingredient lists. For example, ingredient lists will now need to appear in black print over a white or neutral background, and with both upper and lowercase letters, consistent with the Nutrition Facts table. Moreover, the main ingredients (also known as the first-generation ingredients) may voluntarily be separated with bullets (instead of commas) for an improved visual impact.
The figure below illustrates an ingredient list adapted to the new regulatory requirements.
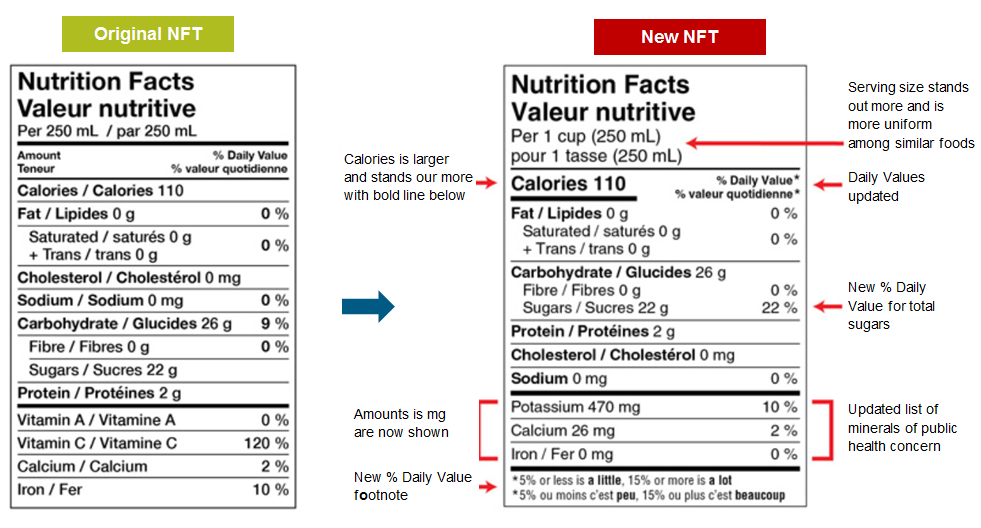
As for the Nutrition Facts table (NFT), the new regulatory requirements include:
- more uniform serving size declarations: serving sizes will now be the consumer-friendly serving measure the closest to the regulated reference amounts. It should also be noted that the reference amounts for several food products have been revised.
- the addition of potassium in the list of core mandatory nutrients.
- the removal of vitamins A and C from the list of core mandatory nutrients, as these are not considered nutrients of public health concern.
- the addition of a percent daily value for sugars.
- the elimination of the daily value for carbohydrates.
- the declaration of the amounts of mineral nutrients (and vitamins, if applicable) in both absolute amounts (e.g. mg or mcg) and as a percentage of the daily value.
- a change in the order of declaration of the nutrients, to group those providing Calories below the declaration of Calories, followed by the other nutrients (sodium, cholesterol, and vitamins and minerals) further below.
- the addition of a footnote at the bottom explaining that 5% or less of a daily value is considered a little, and 15% or more is considered a lot.
The figure below illustrates differences between the original and new Nutrition Facts table (NFT).
Finally, the new regulations allow:
- for approved nutrient content and health claims to be made on prepackaged fresh fruits and vegetables without additional labelling requirements (i.e. without triggering the requirement to display a nutrition facts table on the package).
- a new health claim, subject to several conditions, on the labels of most fresh, frozen, canned or dried fruits and vegetables: “A healthy diet rich in a variety of vegetables and fruits may help reduce the risk of heart disease.”
The new regulations are effective since December 14, but food manufacturers have until December 14, 2021 to destock their current labels, and comply with the new regulatory requirements.
ACC Label will be presenting a seminar on the new regulatory requirements on Thursday, February 9, 2017. Anyone interested in the event, organized by the CIBÎM (Conseil des Industries bioalimentaires de l’Île de Montréal), may obtain information from the CIBÎM. The CIBÎM presentation will be held in French. Upon request, ACC Label will be happy to translate its presentation for English organizations.
Anyone needing assistance with their Canadian labelling needs should fill the “Request a quote” form on ACC Label’s website.
ACC Label
Nutrition is our background, food our passion, labelling our expertise
since 1990
